Therapy Shows Promise in Trials for Hereditary Cancer Patients
An emerging therapy that attacks cancer cells continues to show promise, most recently in two international studies on women who have breast and ovarian cancer and are carriers of cancer-causing mutations particularly prevalent among Ashkenazi Jews.
Two trials tested the experimental capsule olaparib on women whose breast or ovarian cancer had spread to other organs, and who had previously tried at least two other types of chemotherapy. These patients, typically, have built up a stubborn resistance to further treatment. Still, an encouraging percentage responded favorably for a time to the drug.
Both studies featured groups receiving low and high doses of olaparib. Tumors shrank in 11 of 27 women with breast cancer who received the higher dose. The ovarian cancer study experienced similar results, with tumors shrinking in 11 of 33 women receiving the higher dose.
The women in the studies had the added variable of having inherited the BRCA1 or BRCA2 genetic mutations, linked to 5% to 10% of all breast and ovarian cancers. Ashkenazi Jews are at higher risk of carrying these mutations.
Ultimately, the drug ceased its effectiveness for most women, requiring them to seek other treatment options. Yet these were encouraging preliminary results. Olaparib had mild side effects compared to standard chemotherapy, with a much lower incidence of nausea, fatigue and hair loss. It was also taken as a pill — a big deal for cancer patients who endure visits to the infusion suite to get IVs.
“So far I am rather nauseated and sleepy during the day, but it seems to be much easier than the ‘regular’ chemo,” said Ella Lewin, an ovarian cancer patient who just started the therapy, on the higher dosage, at a clinical trial at the University of Pennsylvania. Lewin’s cancer has recurred three times since she was diagnosed six years ago.
Olaparib, made by AstraZeneca, is one of several drugs early in development called “PARP inhibitors.” The poly ADP-ribose polymerase, or PARP, enzyme fixes DNA damage in both healthy and cancer cells. Medicine like Olaparib interfere with, or inhibit, the PARP enzyme, making it even harder for cancer cells with an abnormal BRCA1 or BRCA2 gene to fix DNA damage. These drugs differ from standard chemotherapy in that they don’t appear to harm healthy cells.
“This is one of the ways that modern biology is going to have a greater and greater impact on the cancer problem,” said Dr. Daniel Silver, an assistant professor of medicine at the Dana-Farber Cancer Institute.
Silver, an oncologist who treats breast cancer, said he was excited about the results of the trials, published in The Lancet last month. “Every new tool I have in my toolbox is something else I can use to hold off the progression of the disease longer,” he said.
He referenced the breast cancer study, in which, he said, the average patient responded for a little less than 200 days. “For somebody who, without this, may have been dying very quickly, that’s very significant,” he said. “That’s 200 more days that they have with their family.”
Dr. Mark E. Robson, a co-author of the studies and clinic director of Clinical Genetics Service at Memorial Sloan-Kettering Cancer Center, said the studies benefit from all the genetics research that has been done over the years.
“Without those studies, we wouldn’t have thought to try these drugs in this specific subset of patients,” he said.
This research follows a 2009 study of breast cancer patients involving another PARP inhibitor, iniparib. Half its participants received a combination of iniparib and standard chemotherapy; the other half received the standard treatment alone. The women who got iniparib lived for about 12 months, compared to about eight months for women who did not receive the drug. Iniparib, the first PARP inhibitor to complete phase-three clinical trial, may ultimately be the first to obtain FDA approval.
Dr. Judy Garber, associate professor of medicine at Harvard Medical School and a co-author on the olaparib studies, called the effect of joining a PARP inhibitor with traditional chemotherapy a “double-whammy.”
Of the iniparib data, Garber said, “If you give [PARP inhibitors] with chemotherapy and they work better, maybe this will make chemotherapy more effective for a much broader group of people with cancer that do not have mutations or inheritable forms of cancer. Or, maybe, you give it in the beginning [of treatment] and it’s even more effective than chemo.”
BRCA1 and BRCA2 carriers with a family history of breast or ovarian cancer are asked to consider preventive prophylactic surgery — mastectomy and removal of the ovaries and fallopian tubes. Silver theorized that if the PARP inhibitors are ultimately effective in treating these BRCA tumors, they may even be effective in preventing them.
While cautioning that there is still much research to be done, Silver said, “It may be possible in the future that someone who is a carrier for BRCA1 or 2 might take these drugs for some short period of time — once a year, once a month, on some limited schedule — and that might be enough to substantially decrease their chances of being diagnosed with a malignancy.”
Dr. Harry Ostrer, who is director of the Human Genetics Program at NYU Langone Medical Center and has extensively studied the BRCA mutations in Ashkenazi Jews, called PARP inhibitor research “an interesting new alley.”
Ostrer noted that olaparib clearly had an effect on treating breast and ovarian cancer in both BRCA1 and BRCA2 mutation carriers. While they weren’t cured of their disease, he said, “There was an objective response — their tumors shrunk.”
Robson appears cautiously optimistic about the potential of olaparib. He said that there are a few people in his studies who are continuing to receive the drug and are not seeing progression of their disease.
“It’s another step forward in the targeted therapy arena — defining the unique characteristics of someone’s cancer and going after that specific characteristic to try to get an effective treatment. I think it’s not coming fast enough,” he said, “but it’s coming.”
The Forward is free to read, but it isn’t free to produce

I hope you appreciated this article. Before you go, I’d like to ask you to please support the Forward.
Now more than ever, American Jews need independent news they can trust, with reporting driven by truth, not ideology. We serve you, not any ideological agenda.
At a time when other newsrooms are closing or cutting back, the Forward has removed its paywall and invested additional resources to report on the ground from Israel and around the U.S. on the impact of the war, rising antisemitism and polarized discourse.
This is a great time to support independent Jewish journalism you rely on. Make a gift today!
— Rachel Fishman Feddersen, Publisher and CEO
Support our mission to tell the Jewish story fully and fairly.
Most Popular
- 1

Fast Forward Ye debuts ‘Heil Hitler’ music video that includes a sample of a Hitler speech
- 2

Opinion It looks like Israel totally underestimated Trump
- 3

Culture Is Pope Leo Jewish? Ask his distant cousins — like me
- 4

Fast Forward Student suspended for ‘F— the Jews’ video defends himself on antisemitic podcast
In Case You Missed It
-
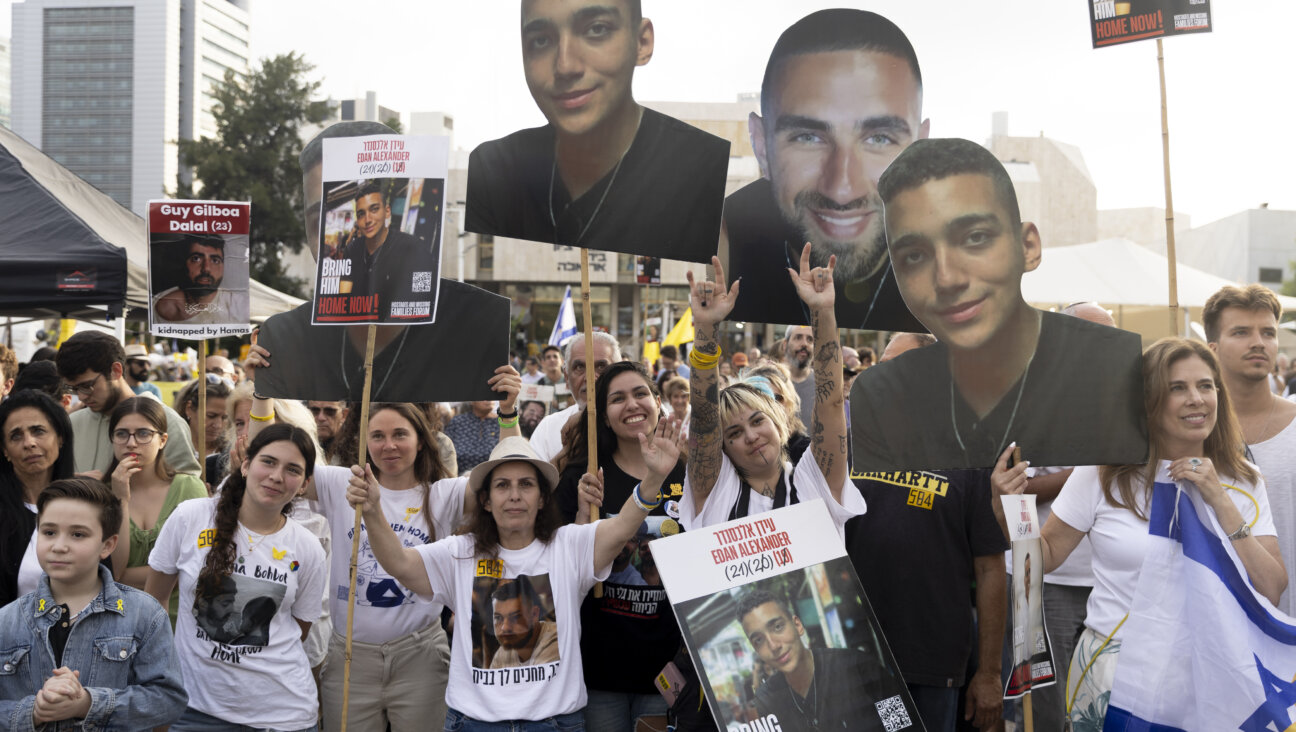
News In Edan Alexander’s hometown in New Jersey, months of fear and anguish give way to joy and relief
-
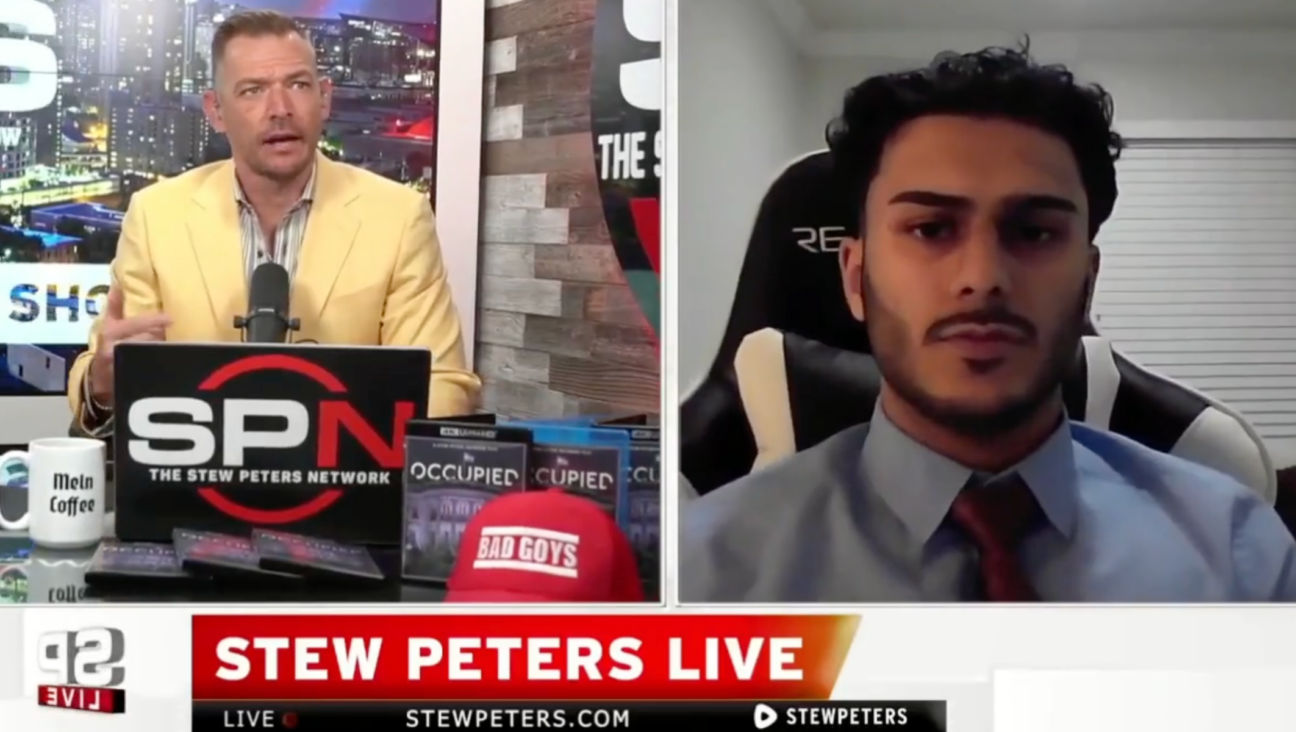
Fast Forward What’s next for suspended student who posted ‘F— the Jews’ video? An alt-right media tour
-

Opinion Despite Netanyahu, Edan Alexander is finally free
-

Opinion A judge just released another pro-Palestinian activist. Here’s why that’s good for the Jews
-
Shop the Forward Store
100% of profits support our journalism
Republish This Story
Please read before republishing
We’re happy to make this story available to republish for free, unless it originated with JTA, Haaretz or another publication (as indicated on the article) and as long as you follow our guidelines.
You must comply with the following:
- Credit the Forward
- Retain our pixel
- Preserve our canonical link in Google search
- Add a noindex tag in Google search
See our full guidelines for more information, and this guide for detail about canonical URLs.
To republish, copy the HTML by clicking on the yellow button to the right; it includes our tracking pixel, all paragraph styles and hyperlinks, the author byline and credit to the Forward. It does not include images; to avoid copyright violations, you must add them manually, following our guidelines. Please email us at [email protected], subject line “republish,” with any questions or to let us know what stories you’re picking up.














